AF and Ozempic
Could the weight loss wonderdrug, Semaglutide (trade name Ozempic or Wegovy) be a treatment for AF?
The Step-HFPEF trial
One of my favourite 'non-AF' trials presented at the European Society of Cardiology Congress last month was 'Step-HFpEF'.
Like Atrial Fibrillation, Heart Failure with a Preserved Ejection Fraction (HFpEF) is a common and debilitating condition. It is caused by a stiffening of the heart muscle rather than a weakness (which would be Heart Failure with reduced Ejection Fraction or HFrEF). It affects 32 million people worldwide, leads to frequent hospitalisations and shortens lifespan. Like in AF, obesity is a big risk factor for HFpEF.
Studies in diabetic patients, and recently in non-diabetics too, have shown that a 2.4mg semaglutide (tradename. Ozempic) injection once a week produces significant weightloss (around 15%). But aside from the weight loss could it improve the symptoms and effects of HFpEF as well?
The Step-HFpEF Trialists took 529 patients with proven HFpEF and typical heart failure symptoms (tiredness, breathlessness, limitation of physical activity) and a BMI of greater than 30kg/m2 (obese) and randomised half of them to receive the semaglutide injections and half to receive a placebo (a dummy injection) for 52 weeks.
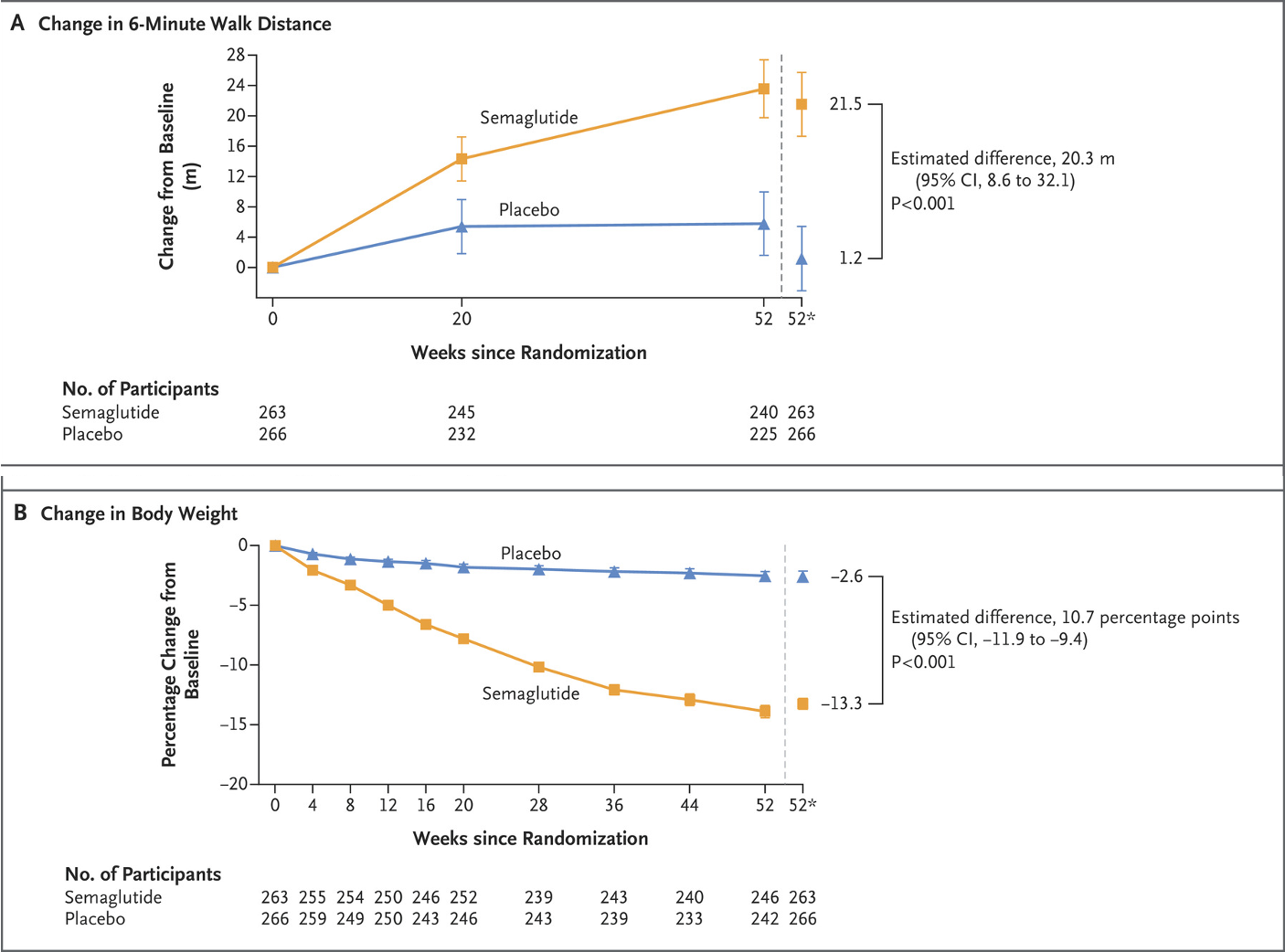
The authors showed that patients who received Ozempic had a significant reduction in their body weight (a 13% reduction versus a 2.6% reduction in the placebo group) and an improvement in their exercise capacity. This is shown graphically in the charts below- but these changes could be down to the weight loss alone rather than an actual improvement in the HFpEF...
However, significant improvements were also seen in their heart failure symptom scores and heart failure hospitalisation rates too, suggesting these aspects of their condition may have benefitted too.
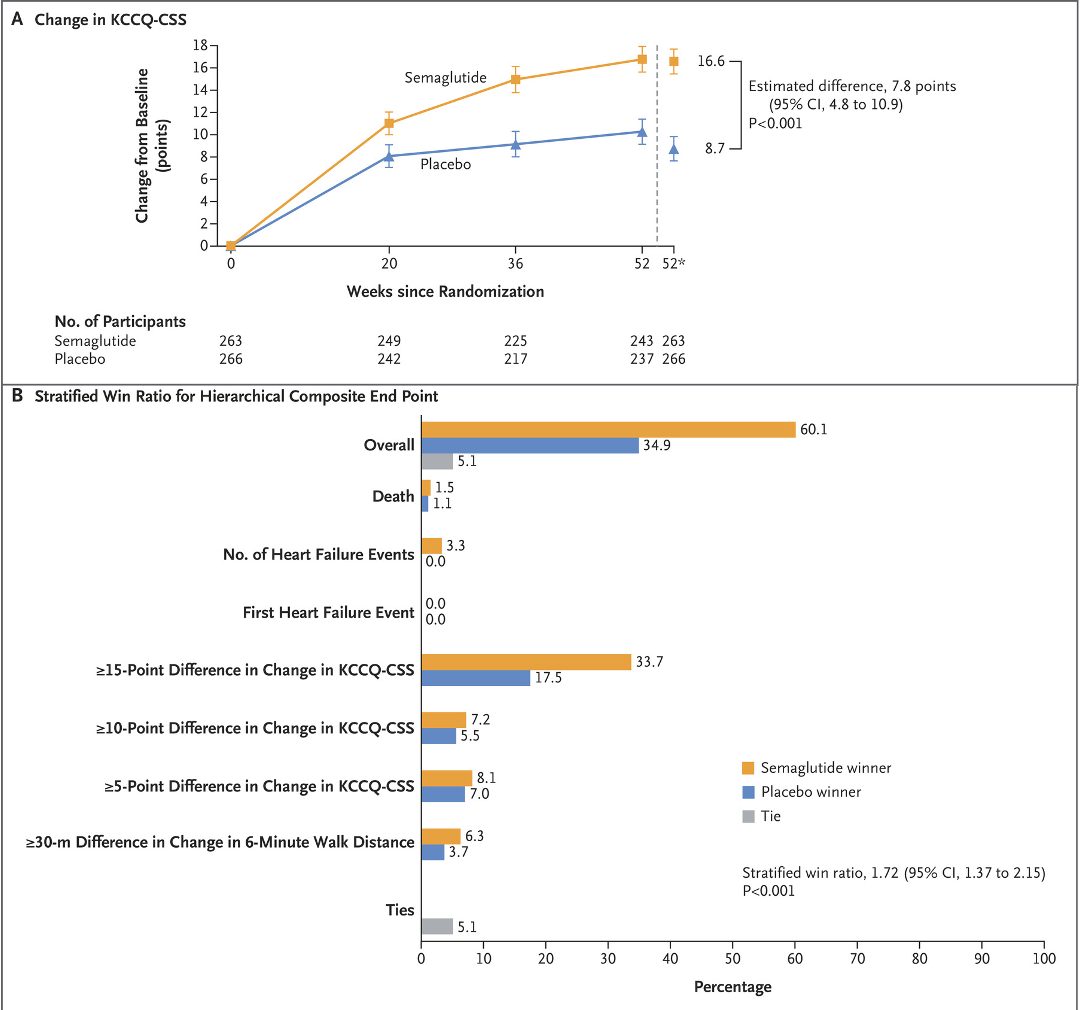
Although the benefits are clear, what remains uncertain is whether all of these benefits was down to the weight alone. Or does semaglutide- a drug that acts to activate the GLP1-receptor found throughout the body, have other direct effects on improving heart failure beyond just the weight loss alone. We have to wait for further studies to understand this better but it looks promising.
So what about a trial in AF?
A similar study was actually proposed in January 2022 in patients with AF! A randomized controlled comparison of 52 weeks of Semaglutide injections versus a placebo in 132 patients with intermittent or early persistent AF was underway in San Francisco, USA. The study had some really interesting exploratory objectives too- they planned to measure the effect on the fatty lining around the heart as well as overall weight and change in AF burden. They were also planning to analyse the effects on the rates of sleep apnoea- a disease commonly found in patients with AF, associated with obesity too. However, the trial was suspended just 9 months in with no explanation given. It is unlikely that any early results would have become apparent The International Clinical Trials database just says 'Company decision'. A similar 100-patient study was documented on the database in 2021 but without results or apparent completion again...🧐
Is there data from other studies?
So if there is no trial of Ozempic in AF patients, what about AF in Ozempic patients?
The initial studies of Semaglutide in diabetic patients also reported the effects on cardiovascular outcomes, including AF occurrence. The rates of AF over the 2-year study period were similar between patients receiving the drug and those receiving the placebo. The numbers are low and the study wasn't designed to look into the AF rates specifically, but nothing abnormal is suggested here.
So what happens next?
We know that obesity is associated with AF progression and worse outcomes- we've discussed that previously here. have data that shows weight loss. We also know that weight loss reverses the natural progression of AF and is associated with improved outcomes in obese patients. The REVERSE-AF study divided 355 patients with AF and a BMI ≥27 kg/m2 according to the weight loss they were able to achieve. All patients were given a personalised weight loss programme including dietary support and counselling.
The 135 patients who lost ≥10% showed significantly less AF progression (going from intermittent to continuous) and significantly more AF reversal (going from continuous to intermittent) than patients who achieved less weight loss.

So if we know Semaglutide leads to 10-15% weight loss in obese patients and if we know 10-15% weight loss leads to greater AF reversal and less AF progression, do we need a trial of Semaglutide in AF patients?
I would argue yes- but not to measure the weight loss patients can achieve (again) but instead to measure any additional benefits (or risks) related to weight loss via Semaglutide itself. We know that one side effect of Semaglutide is that patients can experience a higher resting heart rate. We know that the sinus node (responsible for setting the normal heart rhythm) has GLP-1 receptors (the receptors that semaglutide activates in the gut and pancreas. Although no effect on AF rates has been shown, this hasn't been specifically studied either and given this side effect and the prevalence of AF- it is worth understanding this further and actively demonstrating safety rather than just assuming it.
So let's hope ESC 2024 will feature the STEP-AF trial or atleast intentions towards it.
Kosiborod MN, Abildstrøm SZ, Borlaug BA, Butler J, Rasmussen S, Davies M, Hovingh GK, Kitzman DW, Lindegaard ML, Møller DV, Shah SJ, Treppendahl MB, Verma S, Abhayaratna W, Ahmed FZ, Chopra V, Ezekowitz J, Fu M, Ito H, Lelonek M, Melenovsky V, Merkely B, Núñez J, Perna E, Schou M, Senni M, Sharma K, Van der Meer P, von Lewinski D, Wolf D, Petrie MC; STEP-HFpEF Trial Committees and Investigators. Semaglutide in Patients with Heart Failure with Preserved Ejection Fraction and Obesity. N Engl J Med. 2023 Aug 25. doi: 10.1056/NEJMoa2306963. Epub ahead of print. PMID: 37622681.
Middeldorp ME, Pathak RK, Meredith M, Mehta AB, Elliott AD, Mahajan R, Twomey D, Gallagher C, Hendriks JML, Linz D, McEvoy RD, Abhayaratna WP, Kalman JM, Lau DH, Sanders P. PREVEntion and regReSsive Effect of weight-loss and risk factor modification on Atrial Fibrillation: the REVERSE-AF study. Europace. 2018 Dec 1;20(12):1929-1935. doi: 10.1093/europace/euy117. PMID: 29912366.
